Life Histories of Passionflower-Feeding Flea Beetles
in Northeastern Costa Rica
Contents
Introduction
Methods
Results and Discussion
Species Descriptions:
Disonycha quinquelineata
Monomacra chontalensis
Monomacra violacea
Parchicola "black-tibia"
Parchicola "DF2"
Parchicola "yellow-tibia"
Pedilia "red"
Ptocadica bifasciata
Ptocadica "red"
Ptocadica "yellow"
Table 1. Passiflora x Flea Beetle Interactions Recorded from La Selva
Table 2. Barcode sequences
Table 3. Cyanogenesis in adult flea beetles
Table 4. Aposematic coloration and possible mimicry
Table 5. Larval feeding strategies
Flea beetles (Coleoptera: Chrysomelidae: Galeruciae: Alticini) are diverse in moist tropical environments, with perhaps 1000 species in Costa Rica, Central America alone (Furth, et. al. 2003). Most tropical flea beetle collections have been obtained by sweep sampling, insecticide fogging, and other non-selective means. The great majority of species are known from pinned specimens with little or no life history information, and very few have been studied in their natural environment.
In the 1970's JS collected flea beetles feeding on several species of Passionflower vines (genus Passiflora) at the La Selva Biological Station in the lowland wet forests of Northeastern Costa Rica. The collections were made while sampling Passiflora vines for Heliconius butterfly eggs and larvae in various habitats across the field station, including all the known Passiflora species. The compiled flea beetle collection included about the same number of species as were found in the butterfly community, with both communities having seven common species and 2-3 rarer species. Based on this similarity, Smiley (1982) suggested that species diversity in these two communities might be controlled by their common host plant resource: interaction with the Passiflora host plant community. Smiley (1982) also suggested that flea beetles may have colonized Passiflora more than once, based on the apparent diversity of genera in the community. The lack of a well-supported phylogenetic tree for flea beetles made this difficult to evaluate further.
In 2009 JS returned to La Selva to further investigate the Passiflora-feeding flea beetle community. By 2015 he had accumulated over one year's sampling effort, creating a much more complete picture of the community as well as a larger collection. With the assistence of David Furth of the U.S. National Museum, we applied modern nomenclature to the collection. We also conducted a genetic "barcoding" analysis to help resolve the species, including discovery of an unsuspected "cryptic" species. The community is now nearly as well-defined as the Heliconius butterfly community described in Smiley (1978), improving the basis for comparisons originally made in Smiley (1982). In this article JS presents notes and descriptions of the species in this community, including life-history information.
Flea beetle survey at La Selva. The habitat surrounding La Selva Biological Station offers an excellent ecological matrix for studying insect communities. Year-round rainfall and uniformly warm air temperatures promote intense competition among the 2000 species of plants. The habitat also includes thousands of species of insects and hundreds of species of vertebrate animals, most of which are continuously active in reproduction and survival. In the 1970's JS investigated a community of Passiflora vines at La Selva (Smiley 1978). Passiflora vines are relatively small plants, widely scattered in the massive tangle of vegetation, and often difficult to locate. In 1975, while sampling Passiflora for Heliconius eggs and larvae, JS discovered small numbers of 3-4mm long colorful beetles on the foliage of some of the Passiflora. He began recording these observations and collecting specimens while walking transects. Over time this resulted in a collection that, after examination by staff of the US National Museum, revealed the presence of five species in four genera, with 3-4 more morphospecies without names. The results of this collection formed the basis of a presentation and proceedings publication (Smiley 1982). In 1991 JS returned to La Selva and re-discovered the flea beetles, finding the seven common species in just two days of searching. Then he made another short visit in 2009, and spent several days searching, again finding most of the 1975 survey species. Encouraged by the apparent stability of the flea beetle populations, he returned for slightly longer surveys in 2010 and 2011. Finally, in 2012- 2015, JS carried out a much more thorough survey, observing and recording well over 1000 individuals and their host plants. All months of the year were sampled except May-July.
Flea beetles were sampled by examining individual Passiflora vines for characteristic feeding damage, and, if damage was seen, searching the plant for flea beetles. Beetles often hide under leaves and occasionally on adjacent leaves 5-10 centimeters from the host plant. The best time to search was found to be in the early morning or after rain, when the beetles are likely to be visible, on top of the leaves. Passiflora vines were located by walking trails at the La Selva Biological Station. Trails traverse primary forest including light gaps, forest edge where trees meet disturbed areas, and second growth areas where land has been cleared but is now growing back. The latter includes the successional plots, experimental parcels where the land is regularly cleared on a five year rotation. The La Selva property includes 14 species of Passiflora, 12 of which grow in the lower elevations where JS could readily sample them for butterflies and flea beetles. Adults were counted multiple times on a given plant over a 2-3 month period, but only the maximum count for each species was recorded so as to avoid counting the same beetles twice.
Adult beetles on plants were captured using two ounce polypropylene condiment cups with snap-on lids. Being careful not to touch or shake the plant, the open cup is positioned over the beetle. The lid is then positioned opposite the cup, under the leaf. The leaf (and beetle) are trapped by gently bringing the lid and cup together. When contact is made, the beetle hops into the cup and the cup plus opposing lid are gently slid off the leaf leaving the trapped beetle. Larval beetles were captured by removing their substrate leaf and placing the leaf in a cup. Cups with beetles were brought into the laboratory at La Selva.
Field-collected beetles were either killed by freezing or used in feeding experiments. Killed beetles were either mounted on points and placed in a pinned insect collection, put in 95% ethanol in microcentrifuge tubes for genetic analysis, or preserved in Karnovsky fixative for electron microscope analysis. The pinned insects are then divided into a Costa Rican collection curated by the Costa Rican National Museum, and a research voucher collection for the United States National Smithsonian Museum. Live beetles were handled by cooling them in a refrgerator or by anaesthetizing them with carbon dioxide. Permitting was coordinated by the staff of the Organization for Tropical Studies, and included research and collecting permits, specimen export permits, and a special permit for conducting genetic analysis. Alcohol-preserved specimens were kept frozen (-30ºC.), and Karnovsky fixative specimens kept above freezing at (3ºC.).
Feeding Experiments and Larval Rearing. JS observed and measured the ability of flea beetle larvae and adults to consume alternate host plants within Passiflora. Larvae and adults were tested by placing them singly in a "Glad" brand plastic box approximately 15x20cm x 10 cm deep. Boxes were modified with a 7mm diameter hole in the side, suitable for inserting a 6mm outside diameter tube. When beetles needed to be handled JS would connect the tube to a supply of CO2 and bleed the gas into the plastic box until the beetles stopped moving. To do this he used a large party balloon filled from a laboratory CO2 tank, fitted with a valve. Plant cuttings were trimmed to fit in the box and the stems inserted into small water-filled vials with cotton plugs. Feeding was observed daily and JS recorded leaf area eaten.
Except for Pedilia "red", immature stages were difficult to find. Ptocadica larvae were seen occasionally on P. auriculata and P. biflora (Pt. bifasciata) and on P. lobata (Pt. "red"). Pedilia "red" was brought into the lab for feeding trials. Pt. "red" larvae were obtained by placing the adult Pt. 'red" on a P. lobata plant in the shadehouse, and waiting until eggs were laid and larvae emerged. Egg laying and observation of larvae was also accomplished by placing potted plants in collapsible "Bioquip" cages 60x60cm x 90cm tall. Two to ten flea beetle adults were released in each cage. Cages were checked every 2-3 days for mating behavior, egg placement, larvae and new adults. Unfortunately the potted plants became infested with a 2mm long black ant which may have affected the results by removing eggs, larvae and pupae. When eggs or larvae were seen the potted plant was removed for examination.
Feeding trials and larval rearing cages were kept in the La Selva "ambient" lab which is open to outdoor air circulation and offers temperature and humidity conditions similar to ambient values. Cages with potted plants were maintained in a "shade" house open to the rain and partially shaded to provide good conditions for growth of potted plants.
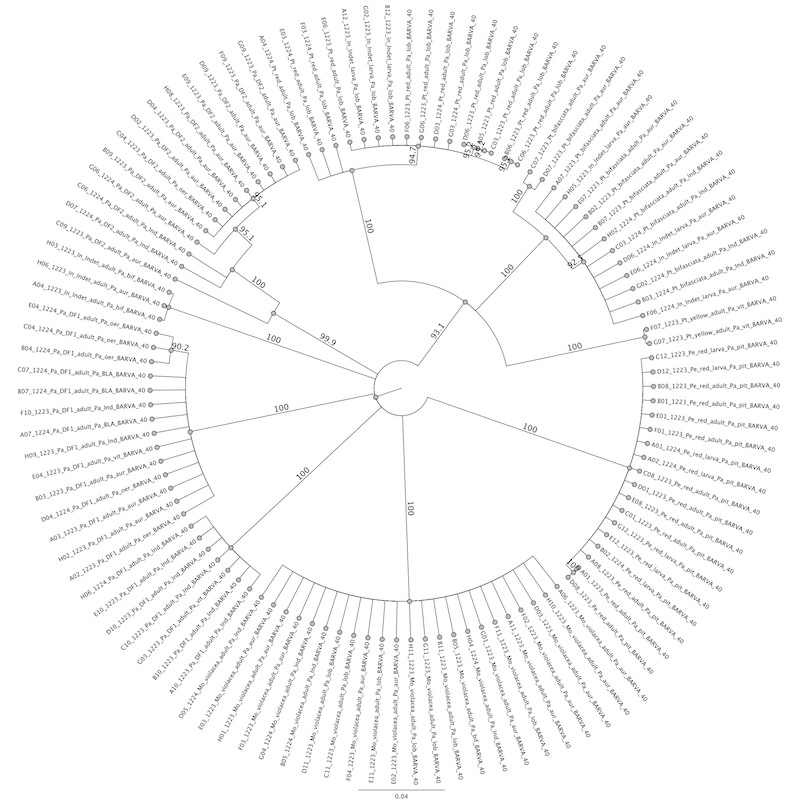
Genetic Analysis. JS placed a representative sample of eight flea beetle species in alcohol-filled microcentrifuge tubes, including 13-23 adults or larvae of each species. For the rarer species this was not possible, for example Ptocadica "yellow" was represented only twice and Monomacra chontalensis and Disonycha quinquelineata not at all. Tubes were kept refrigerated at -30ºC., except during transport and sample preparation. Flea beetle legs or other parts (larvae) were placed in 96-well plates, in buffer solution, and plates were mailed to Carlos Garcia-Robledo at the United States National Museum. Carlos then processed the samples along with other Neotropical Chrysomelidae, using genetic "barcoding" procedures outlined in Garcia-Robledo (2013). The procedures include sequencing Cytochrome Oxidase I (CO I) region of the mitochondrial genome of each specimen. We then used the sequences to create a table of similarities between all individuals as well as using Geneious Pro (v. 5.6.5) software to generate a neighbor-joining tree with bootstrap statistics (100 replicates; see Figure 1). We also created a table of average similarities illustrating the differences between species (Table 1).
Host Plant and Flea Beetle Cyanogenesis Survey. JS used a commercially available "Gasalert Extreme" meter for measuring HCN gas production by Passiflora vines and the Passiflora-feeding flea beetles. JS made over 1250 measurements on crushed leaves of all 12 Passiflora species, as well as adults of six flea beetle species. The technique is sensitive to about 0.05 μM/g of emitted HCN gas and gives repeatable results to within about 10% of recorded values. This work will be presented elsewhere (Smiley, in prep.) but some general results are relevant here in the context of life history information.
More than 500 hours of field observation revealed that flea beetles were sparcely scattered across Passiflora vines at La Selva. Roughly 50% of P. lobata, P. auriculata, and P. biflora were seen to have characteristic flea beetle feeding damage, especially larger plants. Other species were less likely to show damage, and other species were never attacked ( Table 1). The sparse distribution of the vines combined with low probabilities of infestation indicate low population numbers for these 3-4mm long beetles. This parallels the Heliconius butterfly community feeding on the same plants, a relatively stable community characterized by low numbers of individuals (Smiley 1978).
Field observations combined with examination of pinned specimens and genetic analysis revealed 10 species of flea beetle feeding on Passiflora vines at La Selva. Four of these appear to belong to described species: Disonycha quinquelinata, Monomacra chontalensis, M. violacea, and Ptocadica bifasciata. The other six belong to three genera, Parchicola, Pedilia and Ptocadica but have not yet been formally described. Up until now we have given them morphospecies names: Parchicola "black tibia", Pa. "DF-2", Pa. "yellow-tibia", Pedilia "red", Ptocadica "red" and Pt. "yellow". In this paper we formally describe these species, and summarize the life-history information we have gathered from observing them in the field and laboratory. Field observations also indicate that, although other flea beetle species occasionally land on Passiflora vines, they do not feed. Similarly, these 10 Passiflora-feeding species have never been found feeding on any plants other than Passiflora. JS concludes that the Passiflora-feeding flea beetles in Table 2 constitute a distinct ecological community.
Passiflora subgeneric grouping
|
Astrophea/Decaloba |
subgenus Passiflora |
||||||||||||
| Flea beetle species | Passiflora species
|
ARB | ||||||||||||
| Parchicola DF-2 | Yellow-legged | 4 | 56 | 74 | 3 | 1 | 3 | 141 | ||||||
| Monomacra chontalensis | Red-black | 10 |
11 |
1 | 5 |
26 | ||||||||
| Ptocadica "red" | Red-white | 188 |
4 |
3 |
1 |
|
197 | |||||||
| Ptocadica bifasciata | Red-brown-white | 1 |
86 | 38 |
125 | |||||||||
| Pedilia "red" **** | Red Pedilia | 145 |
145 |
|||||||||||
| Disonycha quinquelineata |
Striped | 2 |
16 |
18 | ||||||||||
| Monomacra violacea | Blue | 5 |
279 |
162 |
42 |
24 |
7 |
7 |
00 | 15 |
540 |
|||
| Parchicola yellow hind tibia |
Yellow-tibia |
11? | 2 | >1 | 25 | 2? | 53? | 94? |
||||||
| Parchicola black hind tibia |
Black-tibia |
>2 | 21? | ? | 6? | >5 | 34? | |||||||
| Ptocadica "yellow" | Fat yellow | 6 |
24 |
10 |
41 | |||||||||
| total # observed | 150 | 482 | 0 | 323 | 184 | 1 | 32 | 36 | 57 | 8 | 0 | 86 | 1592 | |
| ***cyanide release from crushed foliage (zer0, Low, Medium, High, Very High) |
VH |
0 |
M |
L |
M |
H | H |
0 |
M |
M |
H |
0 |
||
*data = number of flea beetles seen on each species of Passiflora. ** FB genera and species identified by D. Furth, US National Museum. ID's provisional. Passiflora species abbreviations: VIT=vitifolia; AMB=ambigua;QUA=quadrangularis; MEN=menispermifolia; OER=oerstedii; PIT=pittieri; LOB=lobata; COR=megacoriacea; AUR=auriculata; BI=biflora:ARB=arbelaezii; COS=costaricensis. ***HCN data is based on a survey of over 1250 samples of Passiflora species. ****Pedilia adults found on PIT in laboratory garden. |
||||||||||||||
Barcode Analysis. The genetic "barcode" analysis yielded 110 successful sequences, including all Passiflora-feeding flea beetles except D. quinquelineata and M. chontalensis. Analysis of similarity unambiguously revealed 8 species (Table XX), as did the neighbor-joining tree (Figure X). Only four individuals of one species, Parchicola DF-2, suggested possible within-species variation, and that was minor compared to the gap between species. In all others, between-species differences were an order of magnitude less than that between species. The barcode analysis of Parchicola "DF-1" revealed two "cryptic" species. By examining the barcoded specimens and working back from field notes we were able to verify a distinguishing external character: pigmentation of the hind tibia. We named these morphospecies "yellow-tibia" and "black-tibia".This character in turn led to resolution of host plant differences and a 10% difference in body size. We were able to use this character to differentiate pinned specimens as well as those preserved in alcohol.. With this more complete description of the Passiflora-feeding flea beetle community, JS was able to re-examinine the original pinned collection from 1975. All species except Pedilia "red" were present in the original collection, indicating long term (40 years) stability in the community. Pedilia "red" is rare at La Selva and its absence in the 1975 collection is not surprising. The reason it appears in the modern samples is that well after the 1970's this beetle colonized a large artificially planted Passiflora pittieri, next to the laboratory where it may be easily observed.
| Figure 1 Barcode neighbor-joining tree (click on graph to enlarge) |
 |
Distribution and Social Behavior. Sampling in different seasons revealed effects on flea beetle populations. In particular, sampling in August 2015 after 3 months of rain and clouds produced normal occurrance of the common species, but in reduced numbers. For example, JS could only locate M. violacea on P. lobata plants; in other years and seasons they could be found on a range of species. This is in great contrast to Heliconius butterflies whose populations declined dramatically during the same period, as evidenced by reduced oviposition on Passiflora vines.
Some flea beetle species were often found in groups, including Monomacra, Parchicola and Pedilia species. In contrast Ptocadica and Disonycha were usually seen alone. Ptocadica would often appear to space themselves on different branches of their host plant, as if they were avoiding close proximity. Mating for all species was observed on host plant foliage. Overlap between species was common on shared host plants. Monomacra violacea often occupied Passiflora vines together with more specialized species such as Red Ptocadica (on Passiflora lobata), Parchicola DF-2 (on Passiflora auriculata), Ptocadica bifasciata (also on Passiflora auriculata) and less commonly, Yellow-tibia Parchicola (on Passiflora ambigua). Other combinations include Ptocadica bifasciata with Parchicola DF-2 on P. auriculata, and Monomacra chontalensis with Parchicola DF-2 on P. biflora. Noticeably absent were overlapping species from the same genus. Perhaps diversification of these genera requires reproductive isolation achieved by feeding and mating on different Passiflora species.
Cyanogenesis survey. With the exception of Red Pedilia, flea beetles appear to avoid the most cyanogenic plants and plant parts (see Table 1, bottom row). This contrasts strongly with Heliconius butterflies, which are themselves cyanogenic. Heliconius feed on, and may prefer, the most cyanogenic host plant tissues. Many flea beetles including the Passiflora-feeding species eat small amounts in any given meal, typically only a few square millimeters of leaf. Perhaps this behavior lets them feed on mildly cyanogenic plant tissues by allowing their bodies to process the small amounts of HCN gas produced. Also, as described below in the species' accounts, some flea beetle larvae seem to avoid cyanide poisoning by chewing tissues such as stem or root pith or flower parts. The leaf-eating Ptocadica larvae eat small holes in the leaves, like the adults.
The six species of flea beetles tested were not themselves cyanogenic within limits of detection (see Table 3). This limit was far less than the amount measured from Heliconius. The data below suggests that the flea beetles are at, most only slightly toxic by virtue of HCN released. More likely they release no cyanide at all. However, like Heliconius, they may well be toxic or distasteful by other agents, including sequestration of Passiflora defensive chemicals. No HCN was detected among the flea beetles, in contrast with Heliconius sara. The limit of detection is a function of the volume of the test chamber times the sensitivity of the instrument (lower limit = 0.3 ppm by volume of gas) divided by the pooled weight of the insect sample. There is little information on the threshold for HCN poisoning in insects. However, water containing 0.01μM of HCN per gram was found to be safe (no adverse effects seen) when consumed by rats over a 90 day period (NTP 1993); another study found that rats were not adversely effected with a diet of 0.40 μM of HCN per gram (Howard and Henzel 1955).
Predator Avoidance. Field observations suggest that flea beetles are relatively protected from predation. They use visual cues to watch for approaching predators in addition to detecting movement of the host plant leaf they are on. When they become alerted their escape behavior is to hold in place with legs in spring position until actually jostled or touched by a predator, and then to spring away with tremendous acceleration (10 to 271g, depending on the species; Brackenberry and Wang 1995), opening the wings 10-20 cm in flight, and continuing with wing powered, controlled flight. Controlled flight, sometimes including hovering, is then used to examine the surrounding vegetation. Landing follows .5 to 1m away, usually off the host plant. Scars are often seen on the relatively soft cuticle of the elytra of the adult beetles. These appear to fit the mandibles of predators such as Ectatomma species, suggesting that beetles often escape when attacked by these large predatory ants.
Larval flea beetles also appear resistent to some types of ant predation. The leaf-eating larvae of Ptocadica are covered with rounded protuberances covering the entire larva including the head and legs. Their shape is similar to the larvae of Lycaenid butterflies, well-known to be resistent to handling by ants. They often walk on stems patrolled by multiple ants. JS also confined 7 Red Ptocadica larvae with Ectatomma tuberculatum in a one liter chambers for 13 days: the larvae were not bothered even though the ants were only fed sugar water. The larvae of Parchicola discovered to date feed on the stems and perhaps roots of their Passiflora host plants, a location where contact with ants would be frequent. They possess tiny round organs on the tips of their rounded protuberances. Their function is unknown, but they may play role in anti-predator behavior. These will be described further below in the species' accounts.
Coloration. The Passiflora-feeding flea beetles possess brilliant coloration, perhaps enhanced by a relatively soft transparent cuticle that dampens reflections and highlights. The red/white/orange/yellow colors rapidly dissappear upon death unless special precautions are taken to preserve them. However the dark blue/brown colors remain, providing some clues as to species' identity in pinned collections. Vivid colors suggest a possibly aposematic function, maybe warning potential predators of the beetles' undesirability as prey. Perhaps the colors advertize their escape ability? Another possibility is that bright colors may assist in social behavior, perhaps facilitationg aggregation. Table 4 summarizes the color characteristics, including the possibility of color pattern convergence and/or mimicry. Mimicry seems most likely for two species of Ptocadica. Pt. "yellow" could easily mimic the Parchicola "yellow-tibia" on Passiflora ambigua or the Pa. "black-tibia" on P. vitifolia, plants that it shares with those species. Pt. "red" could mimic Pedilia "red"; even though they are on different plant species (P. lobata and P. pittieri, respectively) they share the forest habitat. Ptocadica bifasciata also resembles another flea beetle that appears to feed on Convolvulaceae vines. It is likely that Passiflora-feeding flea beetles, like Heliconius butterflies, are dynamic in evolution of color forms. For example, the community of Passiflora-feeding flea beetles at Corcovado National Park in southwestern Costa Rica is quite different-looking from those in Table Y.
Larval feeding As may be seen below in Table 5, the Passiflora feeding flea beetles at La Selva can be divided into two larval types: root/stem feeders and leaf feeders, with approximately the same number of species in each group. JS has found that immature forms for three of the five closely related Monomacra and Parchicola species are very similar, with long slender cylindrical eggs and slender, fragile larvae dotted with tiny setae. The setae are themselves modified into even smaller outward-projecting spheres, that are placed in such a way as to offer a chemical defense against predators. Eggs are laid on the underside of old leaves by the base of the plant (Pa. "yellow-tibia"), or on rootlets in the soil adjacent to the host plant ("DF2" or M. violacea). In contrast, the other 5 species' juvenile forms are not appear to be associated with the host plant roots. Based on observations on Pt. bifasciata and Pt. "red", Ptocadica species lay ovate eggs on the host plant leaf surface. These hatch directly into leaf-eating larvae. Pedilia lay long cylindrical eggs, like Monomacra/Parchicola, but these are places in long rows end to end on the leaf surface and also hatch into leaf-eating larvae. In these 4 cases the larvae have rows of rounded bumps dorsally that appear thickened and that cover the head effectively, but which lack any setae. In the case of Pedilia, once the larvae grow for a few days they switch to stem feeding, eating the green epidermis layer off the stems. The larvae of D. quinquelineata look somewhat like those of Pedilia, except that the modified setae/sphere organs are present, although with a more elongated shape.
Disonycha quinquelineata ("Five-striped" flea beetle)
This species is rare at La Selva, seen a few times in the 1970's on Passiflora biflora and P. auriculata in the garden area (now the lab clearing), in the successional plots in 2012 on biflora, and on the edge of the La Selva property near the Arriera-Zompopa cabins. The largest of the La Selva Passiflora-feeding flea beetles, the females often have their abdomen distended with eggs as they feed on the host plant. JS has often encountered this species on P. biflora outside La Selva, for example in Tortuguero, Costa Rica and Bocas del Toro, Panama.
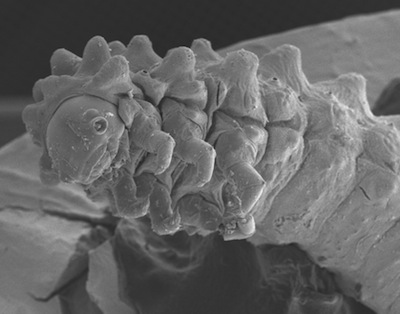
The larvae are reddish-brown and are studded with hundreds of small glandular-appearing "balloon" organs (see photos below). Larvae look very much like the larvae of Parchicola "yellow-tibia" (YTL), and suggest possible relatedness between Disonycha and Parchicola. JS has not yet seen the eggs of this species. Larvae feed on the leaves of the host plant.
The larvae feed on leaves and new growth of P. biflora. Like other Passiflora-feeding flea beetles, the larvae are covered with rounded protuberances on the back and sides, covering the head and legs almost totally. The protuberances are tipped with 1-3 tiny flask-shaped organs. The rounded ends are patterned and seem to be made of cuticle. The number of organs is related to the size of the protuberance. They look related to organs on the Parchicola, except for being elongated. The bases of the organs are black, surrounded by a separate black ring. The function of these organs is unknown but they look capable of disseminating chemicals to potential predators such as ants and wasps. The head is covered with a dense "crown" of smaller protuberances with single tip organs. The tail end covers the powerful anal clasping organ which enables the larva to grasp the substrate during rest and locomotion; the organs are found there as well.
Monomacra chontalensis (Red and Black Flea Beetle)
This species is uncommon, but has been found on a wide range of Passiflora subgenus Decaloba species, including P. biflora, P. lobata, P. arbelaezii and P. costaricensis. The latter two species are highly cyanogenic, suggesting that M. chontalensis may be more tolerant of HCN release than many other flea beetles. Alternately, it may possess some other mechanism to neutralize and prevent HCN release. This species may be very long-lived. JS collected 2 adults of unknown age from the wild on 2 December, 2013, and at least one lived on a potted P. biflora in the shade house until 18 March, 2014 (106 days!).
Like M. violacea, this species is readily recognizable in museum collections by the shiny black band across the abdomen, even when the red coloration is lost.
JS has not found eggs or larvae of this species, but is probably very similar to its congener M. violacea (see below).
|
|
|
| Monomacra chontalensis |
Monomacra violacea (Blue Flea Beetle)
The blue flea beetle may be found eating holes in the leaves of most Passiflora species, often in groups of 5-15. Host species include some highly cyanogenic species such as costaricensis and pittieri, indicating that M. violacea, like its congener M. chontalensis, may be able to tolerate cyanide-releasing chemicals in its diet. Its wide distribution at La Selva is somewhat like Heliconius cydno, the most "generalist" of the La Selva heliconiines. Also like H. cydno, this species is frequently found in forest light gaps and clearings as well as forest edge habitats. Unlike H. cydno, M. violacea seems highly successful on P. lobata, the Passiflora with hooked trichomes. In fact, most P. lobata "infested" with a population of M. violacea die within 6 month to a year.. This species may be partly responsible for the high demographic turnover rate for P. lobata.
Like genus Parchicola, Monomacra has a flattened, incised rectangular area on the hind edge of the pronotum. It is difficult to see in these photos because of the reflectivity of the cuticle. This species is readily recognizable in museum collections by the shiny blue cuticle.
JS has not yet found larvae of this species - he suspects they are basal stem feeders or surface root feeders like their Parchicola relatives. The caged population in the shadehouse was isolated with P. vitifolia for 2 months, and with P. lobata for 2 more months, but was not found to lay eggs or reproduce. However, the potted plants were infested with small ants that may have eaten the eggs and larvae.
Common in all seasons of the year, often in groups of 10-20 on a single host plant. Mating takes place on the leaves of the host plant.
Parchicola "black tibia" (Black Tibia Flea Beetle)
The Black-Legged Yellow flea beetles (Parchicola DF1) include two of the most common flea beetle species found on subgenus Passiflora. Genetic "barcode" analysis of 21 individuals revealed two "cryptic" species-level taxa within the Black-legged Yellow designation (14 in one species and 7 in the other). Reviewing the collection history and host plant origin of the sequenced individuals, JS was able to associate one of the species with apparently larger individuals collected from P. vitifolia and P. quadrangularis. P. oerstedii, the most common YBL host, was conspicuously absent from this group. To test the idea that the two taxa came from different host species, JS collected 5 fresh individuals from P. vitifolia and 5 from P. oerstedii. As predicted, JS found the P. vitifolia set to be longer (x=3.52mm; n=5) than the P. oerstedii set (x=3.22mm; n=5; p<0.02; t-test). However, what is more interesting is that JS found all five P. vitifolia individuals to have solid black (or dark brown) hind tibia and all five P. oerstedii individuals to have yellow (unmelanized) hind tibia. This morphological character is preserved in dried specimens and JS was easily able to sort pinned Parchicola DF1 specimens into two groups. Later, upon re-examination of the specimens used in the genetic analysis, JS was able to confirm that the two groups may be distinguished by the color of the hind tibia and that the species may be identified from this external, permanent morphological character.
Working backwards with known specimens JS listed host plant occurrances for both species including many specimens collected in 1975-76. These can be seen in Table 1. Note that both P. "yellow-tibia" and P. "black-tibia" have been found on P. vitifolia, P. quadrangularis and P. oerstedii, and both may also rarely be found on species of Decaloba such as auriculata. This pattern is analogous to Heliconius hecale and H. ismenius, preferring subgenera Passiflora and Distephana, but sometimes using Decaloba. It may be that, as proposed for Heliconius, the ability to consume subgenus Passiflora pre-adapts the species for eating many Decaloba species.
The black-tibia species appears to be most common on P. vitifolia at La Selva. Perhaps it reproduces on that species, analogous to yellow-tibia reproducing on P. oerstedii. In August 2015, after an unusually intense rainy period lasting since June, this species was found commonly, including a group of five on one P. vitifolia shoot. It has also been found during the drier parts of the year.
Parchicola "DF-2" (Yellow-legged Flea Beetle)
This species occurs primarily on P. auriculata and P. biflora, the two most common species of Passiflora subgenus Decaloba. A small but significant number (about 10%) have also been seen on P. lobata (also in Decaloba), a plant whose foliage is protected by hooked trichomes. A rare few (< 5%) have even been seen on P. vitifolia and P. oerstedii, species outside of Decaloba but which produce minimal amounts of HCN gas when crushed.
Until 2011 this and the Black-legged Yellow flea beetle (Parchicola DF1) were grouped together as one species "yellow Monomacra." This erroneous grouping contributed to the perception that these were a single Passiflora-generalist species, when in fact we believe there are at least three species. This one with yellow legs appears to be specialized to feed on Decaloba, while the other two (with black legs) are more generalist but with preference for subgenus Passiflora, including P. oerstedii (Parchicola "yellow-tibia") and P. vitifolia (Parchicola "black-tibia"). However, genetic "barcode" analysis suggested possible additional complexity within Parchicola "yellow-legged" (DF2). Four of the 13 individuals sampled were slightly dissimilar from the remaining 9, with similarity indices ranging from 90 to 96% instead of the usual 98 to 100% among members of a species. However, those four did not group together and so probably do not represent a single separate species. As well, the similarity of 90 to 96% is higher than that between the other Parchicola species, which are between 80 and 84% similar.
A captive YYL "yellow-legged" population in isolation cage D laid at least one batch of 20 eggs on tiny rootlets just below ground level, on P. biflora, and 6 weeks later, on P. auriculata. They look similar to the eggs laid on the underside of P. oerstedii leaves by Parchicola "yellow-tibia". Larvae were not found on the P. biflora batch, but there may have been some new recruitment of adults later. The P. auriculata batch hatched into tiny first instars about the size and shape of the eggs. These were seen crawling along the tiny rootlets.
I expect the later stage larvae to be light colored with many protuberances, each of which are tipped with tiny spherical "balloon" organs, as may be seen on the web page for "yellow-tibia".
| Top view of Parchicola DF2, the "yellow-legged" flea beetle, feeding on Passiflora auriculata. | Compare "yellow-legs" (center) to Parchicola "yellow-tibia" (on left). The third Parchicola, not shown here, is Pa. "black-tibia". |
 |
|
| Eggs of Parchicola DF2, laid on P. biflora rootlets near ground level. Click on photo to expand and see how many are in view. (look closely: there are at least 18 eggs.) | The eggs look identical to the eggs of Parchicola "yellow-tibia". Although JS have not found larvae of this species, JS predict that they will be similar to the larvae of Pa. "yellow-tibia". |
| A Parchicola DF-2 egg about to hatch. Note the black rings where the folded balloon-like organs are appressed to the inside of the shell. These eggs were laid on the rootlets of Passiflora auriculata. | An empty egg shell. The eggs all hatched after JS placed their rootlet in a humid box with vermiculite, and covered with a moistened paper towel. |
| New first instar YYL larva hatched in the previous 24h. Not yet fed. | The tiny "balloon" organs appear deflated and darkly pigmented as compared with the lighter-colored organs seen on older instar "yellow-tibia" larvae. |
| Parchicola "yellow-legs" on P. biflora | Parchicola "yellow-legs" on P. costaricensis flower, eating filaments. Note M. violacea on flower as well. |
 |
 |
 |
 |
Parchicola "yellow-tibia" (Yellow-tibia Flea Beetle)
The Black-Legged Yellow flea beetles (Parchicola DF1) include two of the most common flea beetle species found on Passiflora subgenera Passiflora and Distephana. Genetic "barcode" analysis of 21 individuals revealed two "cryptic" species-level taxa within the Black-legged Yellow designation (14 in one species and 7 in the other). Reviewing the collection history and host plant origin of the sequenced individuals, JS was able to associate one of the species with apparently larger individuals collected from P. vitifolia and P. quadrangularis. P. oerstedii, the most common YBL host, was conspicuously absent from this group. To test the idea that the two taxa came from different host species, JS collected 5 fresh individuals from P. vitifolia and 5 from P. oerstedii. As predicted, JS found the P. vitifolia set to be longer (x=3.52mm; n=5) than the P. oerstedii set (x=3.22mm; n=5; p<0.02; t-test). However, what is more interesting is that JS found all five P. vitifolia individuals to have solid black (or dark brown) hind tibia and all five P. oerstedii individuals to have yellow (unmelanized) hind tibia. This morphological character is preserved in dried specimens and JS was easily able to sort pinned Parchicola DF1 specimens into two groups. Later, upon re-examination of the specimens used in the genetic analysis, JS was able to confirm that the two groups may be distinguished by the color of the hind tibia and that the species may be identified from this visible, external morphological character.
Working backwards with known specimens JS listed host plant occurrances for both species including many specimens collected in 1975-76. These can be seen in Table 2. Note that "yellow-tibia" are dominant on P. oerstedii, but may also be rarely found on P. vitifolia, P. quadrangularis and even more rarely on species of Decaloba such as auriculata. This pattern is analogous to Heliconius hecale and H. ismenius, preferring subgenera Passiflora and Distephana, but sometimes using Decaloba. It may be that, as proposed for Heliconius, the ability to consume subgenus Passiflora pre-adapts this species for eating Decaloba species.
Parchicola species have an impressed, thin rectangular area on the hind part of the pronotum (barely visible in one of the photographs on the BTL page). This, with their fairly long, parallel-sided elytra, separate them from the rounded, dome-shaped species of Ptocadica and Pedilia. David Furth assigned the temporary name "DF1" for one of these yet-to-be-described species, but it is not clear whether that morphospecies name belong to YTL or BTL. Like nearly all of the species described here, these specimens lose their vibrant color and become dull brown after death. However, the tibia color difference remains.
These flea beetles became rarer during the rainy season in November and December 2012, and then rose again in March and April, 2013. They have been consistently common in late 2013 and early 2014. In August 2015 after two months of intense rainy conditions, JS did not see any on P. oerstedii or P. ambigua.
Over 50% of all YTL observations are from P. oerstedii, and populations of YTL in isolation cages with this plant have been found to reproduce. In an attempt to isolate and describe the juvenile stages of this species, 9 adult YTL were put in a 2x2x3 foot cage with 2 potted P. oerstedii plants. This was done in early December, 2013. By January 11, 2014, new adults were emerging into the cage. Close examination of the potted plants revealed eggs under the older leaves, larvae feeding on the basal stem near ground level, and one pupa in the soil beneath the stem. The eggs were laid in groups of 8-12, arranged at random. They are very long and thin (about 5:1 ratio), and have a textured surface. The larvae have the same "hill and valley" rounded protuberances as other flea beetle larvae (Red Ptocadica, for example), except that at the crown of each protuberance there are 1-3 tiny spheres on short stalks. The spheres are patterned and seem to be made of cuticle; they are not liquid droplets (althoughthey may contain liquid). The function of these seems to be unknown. Larvae probably feed on the leaves where they are hatched. Later they apparently (I have not yet seen this) walk down the stem toward the base of the plant and begin feeding on the stem. They chew the green epidermis of the stem as well as the woody parts. In this respect these larvae are similar to those of Pedilia, which are also stem feeders in the 2nd and 3rd instars.
 |
|
| Parchicola "yellow-tibia" feeding on P. ambigua. The yellow tibiae are not easily visible in this photo. | Here the orange-yellow hind tibia are clearly visible. In life, these shiny beetles appear more yellowish than orange. |
| The hind (meta-) tibia of Pa. "yellow-tibia" are primarily yellow, unlike Pa. black-tibia shown at right. The fore and middle tibia are always black, distinguishing this species from the yellow-legged Parchicola DF2 with no black leg parts. | Pa. "black-tibia" for comparison. |
| Parchicola "yellow-tibia" eggs, <1mm long | 3rd instar Parchicola "yellow-tibia" |
| Close up of tiny spheres | Larvae feeding on P. oerstedii stem near base of plant |
 |
|
| Parchicola "yellow tibia" larva feeding on stem of P. oerstedii. | This Parchicola DF2 larva is newly hatched from the egg. The new 1st instar "yellow-tibia" larvae probably look just like this. Note that the "tiny spheres" look pigmented but also deflated and folded rearward . |
|
|
|
| Parchicola "yellow-tibia" pupa, found in soil at base of plant. This pupa was bright yellow. | |
This species appears to specialize on P. pittieri only. According to Erika Deinert it appears to be nearly identical (except for color) to the Pedilia sirena found on the Osa Peninsula in Southwestern Costa Rica. That species, an orange-yellow color, is also a specialist on P. pittieri. A few orange yellow individuals have been found on P. pittieri at La Selva, supporting the idea that these may be one species with two color morphs. The larvae also seem to vary in color, possibly depending on pigments in the host plant stems, and even the frass may be reddish brown on some stems (see photos below). Also, Yellow Ptocadica looks a lot like the yellow form of Pedilia that feeds on P. pittieri. Ptocadica are somewhat longer with a longer pronotum, and the legs and basal antenna segments are melanized. Pedilia legs and first antenna segments are clear and unpigmented.
Red Pedilia adults may be consistently found year-round on the large pittieri plant that grows next to the lab buildings at La Selva. This plant, a remnant of the Passiflora garden we planted many years ago, is large and robust, and supports a large colony of Ectatomma tuberculatum ants. The plant has between 20 and 50 adults present at any given time, and they may often be found feeding on the new growth shoots that erupt from the plant every few days. Adults and young larvae prefer the light green, waxy, soft new growth, and the youngest full-sized leaves are often peppered with feeding holes (adults) or scrapings (larvae). Measurement of HCN production by crushed leaves tells us that leaves of this age have about 5 micromoles HCN per gram leaf tissue (mM/g), and that the amount rises to about 20 mM/g as the leaves age. After about one month the leaves begin to harden and, even though cyanogenesis drops to 5-10 mM/g, the leaves become less palatable. However, at this time the epidermis of the stem and petioles takes on a greatly reduced HCN content, and becomes preferred for the adults and larvae. It is possible that, by scraping the lower leaf tissue, the larvae and adults may avoid release of HCN. Adults mate on the host plant. JS have seen Pedilia feeding damage on some of the wild P. pittieri plants at La Selva, even though JS have not found live beetles.
Larvae are more cylindrical and elongate than Ptocadica larvae, though the protuberances are similar in size. Also, there are no balloon organs, also like Ptocadica but unlike Disonycha.
Feeding trials in which Pedilia larvae were placed in containers with different Passiflora species reveal Pedilia to be a feeding specialist. The only species they would eat besides P. pittieri were P. biflora, P. arbelaezii, and P. costaricensis, and they fed poorly on those. When placed on P. lobata, Pedilia larvae became impaled on the hooked trichomes and died.
| These flea beetles are large (6mm long) and most are very bright red. | The entire body is bright red also, except for the black eyes and brownish antennae. |
 |
|
| Red Pedilia like to cluster together on a leaf, often on the underside in sunny weather. | egg string on light green waxy young leaf of P. pittieri. |
| A denser aggregation. | In two casesI have found Orange-yellow individuals on the plant. Could the different colors reflect diet differences? |
| The only other species of flea beetle JS have found on P. pittieri is the Blue flea beetle, Monomacra violacea. | Pedilia larvae feeding on new leaf. |
| Newly hatched larvae on leaf. Note the feeding pis where the larvae have scraped away the leaf parenchyma. | Older larvae prefer to feed on the epidermis of the stems and petioles. Often, they chew through the petioles, causing the leaves to drop. JS have seen this feeding damage on P. pittieri plants in the forest at La Selva, indicating the presence of this species. |
 |
|
| The stem-feeding larvae generate reddish, fibrous frass. Does the red color of the larvae and adults result from this diet? Are La Selva P. pittieri plants different from those on the Osa Peninsula? | See how yellow these larvae are. They have fed exclusively on young leaves. |
| Pedilia larvae have a shape similar to that of the Disonycha larva; except that there are no bottle-shaped organs tipping the protuberances. | Like other flea beetle larvae, Pedilia must have strong jaws to eat such tough, fibrous food. They often feed in groups of 2-20 individuals. |
 |
 |
Ptocadica bifasciata (Red-brown-white Flea Beetle)
This Ptocadica species feeds primarily Passiflora auriculata and is occasionally found on other Decaloba (on P. biflora 15% of the time; once on P. lobata). Like other Ptocadica, bifasciata often sits under leaves or on foliage next to the host plant. A good flier, bifasciata can hover and examine the foliage before alighting. As shown in the photo below, they sometimes scrape the leaves when feeding. Unlike many of the other species of Passiflora-feeding flea beetle, Ptocadica bifasciata is seldom seen in groups. Typically each beetle will be on their own branch, or be seen sharing a branch with other species such as Parchicola DF-2 or Monomacra violacea.
Larvae of Pt. bifasciata may be seen feeding on leaves and walking the stems. JS have not yet seen eggs, but predict they will be round and laid on the undersurface of the host plant leaves (like red Ptocadica). This species also shows variability in the amount of dark pigmentation, as may be seen in the photos below. Genetic barcoding suggests that these forms belong to the same species. The white and rose colors turn brown in dried museum specimens, but the dark melanic spots remain.
I have found P. biflora plants with multiple individuals of Pt. bifasciata, suggesting that this species can reproduce on P. biflora. This should be confirmed, however.
In our early collections, this species was listed as "Strabala sp. red-brown-white".
|
|
| Feeding on the preferred host P. auriculata. | Scraping the upper leaf surface of P. auriculata. |
 |
|
| Pt. bifasciata sharing P. biflora leaf with M. violacea. | Note paler, smaller dark spots on this individual as compared with photo above this one. Genetic barcoding did not distinguish these two color forms. |
 |
|
| Pt. bifasciata larva on P. auriculata | Side view of larva walking on stem. These larvae have been seen walking on stems with a stream of small ants, with no ill effect. |
 |
 |
| Closeup views of Ptocadica bifasciata. | |
 |
Ptocadica "red" (Red Ptocadica)
This species is commonly found on P. lobata(over 90% of sightings), often in the company of the Blue flea beetle Monomacra violacea. Less social than M. violacea, it often sits singly on its own branch under leaves.This species colonizes a high proportion of P. lobata vines, which in turn are quite short-lived as compared with other Passiflora. This suggests that red Ptocadica may be unusually adept at dispersing and finding new host plants. On one occasion JS found a red Ptocadica feeding on the petiolar nectar gland of a Passiflora ambigua leaf. It was gone when JS returned the next day.
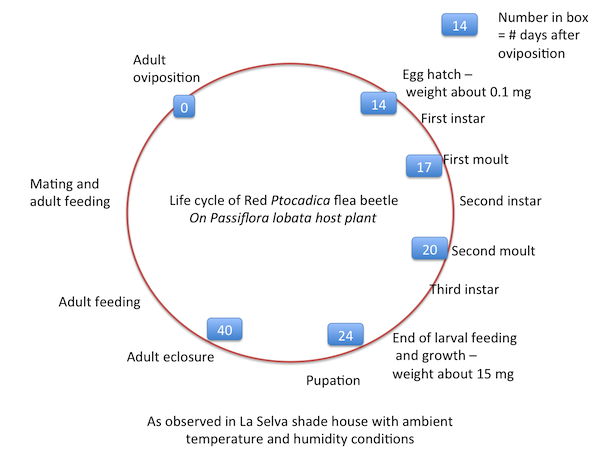
Young larvae feed on the new leaves of lobata and are yellowish brown in color. They seem unaffected by the host plant's hooked trichomes, which kill the non-adapted larvae of red Pedilia. They often moult from the second to the third instar underneath a horizontal stem. Moulting takes a full day or sometimes nearly two days before the larvae resume feeding. Larvae pupate in the soil under the plant. The life cycle (see photos & diagram below) from oviposition to pupal eclosure is at least 40 days, maybe as many as 50 days. This is a much slower development time as compared with a Heliconius butterfly. Adults are fairly long-lived, living about a month in the isolation cages.
After death this species turns brown and, after a few months, becomes visually indistinguishable from the Yellow Ptocadica flea beetle. In museum collections these two species have the appearance of Ptocadica straminea.
Ptocadica "yellow" (Yellow Ptocadica)
Found primarily on subgenus Passiflora (vitifolia, ambigua and oerstedii), this species has become quite rare at La Selva. In 2010-15 JS found fewer than 10 of these beetles. On one occasion JS found a Red Ptocadica flea beetle on a leaf of P. ambigua (an unusual occurrance) next to a Yellow Ptocadica. The Red-white beetle was chewing a hole in the leaf petiole! Like its congeners Pt. bifasciata and Red Ptocadica, JS expect this species to oviposit round eggs and for the larvae to eat foliage rather than stems. JS predict the larvae will look like their congeners (see below) and will be found on subgenus Passiflora.
Yellow Ptocadica looks a lot like the yellow form of Pedilia that feeds on P. pittieri. Ptocadica are somewhat longer with a longer pronotum and the front and middle legs and basal antenna segments are melanized. Pedilia basal antenna segments are clear and unpigmented, as are the legs. Pedilia also has eyes that are strongly lunate, curving around the bases of the antennae. The species also looks like black-tibia Parchicola. Ptocadica eyes are ovate with the small end pointing to the top of the head, and are convex in outline. Parchicola have symmetrical oval eyes that are slightly notched to accommodate the antennae. Parchicola also has the unique flattened rectangle "crease" at the posterior end of the pronotum, although this may be difficult to see in life. Ptocadica lacks this feature.
After death this species turns brown and, after a few months, becomes superficially indistinguishable from the Red Ptocadica flea beetle. In museum collections these two species have the appearance of Ptocadica straminea.
| Like its congeners, Yellow Ptocadica has a wide oval body shape. | In this side view you can see the the domed body shape. This and the larger size differentiate this species from the yellow Parchicola species. Note the all black antennae, unlike those of Pedilia which have a clear first segment. |
| Larva of Red Ptocadica. The Yellow Ptocadica larvae probably look like this. | Closer view emphasizing domed shape. |
| Ventral view, showing oval body shape. Hind legs not melanized, but front and middle tibiae and tarsae are pigmented. | Head and pronotum of Yellow Ptocadica. Eyes are well clear of antennae, and are ovate and convex along their margins. Ovate eyes larger at the bottom than the top. |
IN PROGRESS
Brackenbury, John and Richard Wang. 1995. Ballistics and visual targeting in flea beetles (Alticini). Journal of Experimental Biology 198:1931-1942.
Furth, D. G., J. T. Longino, & M. Paniagua. 2003. Survey and quantitative assessment of flea beetle diversity in a Costa Rican rainforest (Coleoptera: Chrysomelidae: Alticinae), pp. 1-23. In: Special Topics in Leaf Beetle Biology: Proceedings of the Fifth International Symposium on the Chrysomelidae. (D. G. Furth, Editor). Pensoft Publishers, Sofia-Moscow.
García–Robledo, C., D.L. Erickson, C.L. Staines, T.L. Erwin and W. J. Kress. (2013). Tropical plant – herbivore networks: reconstructing species interactions using DNA barcodes. PLOS ONE 8(1): e52967. doi:10.1371/journal.pone.0052967.
Howard, JW and RF Henzel. (1955) Chronic toxicity for rats of food treated with hydrogen cyanide. J. Agric. Food Chem. 3 (4):325-329.
National Toxicology Program (1993) Technical report of toxicity studies of sodium cyanide administered in drinking water to F344/N rats and B6C3F1 mice. National Institutes of Health Publication 94-3386.
Smiley, J. T. 1978. The host plant ecology of Heliconius Butterflies in Northeastern Costa Rica. PhD Thesis, Univ. of Texas at Austin
Smiley, J. T. 1982. The herbivores of Passiflora: Comparison of monophyletic and polyphyletic feeding guilds, in: Proc. 5th Internationial Symposium on Plant-insect Relationships, Wageningen 1982, Pudoc, Wageningen 1982.